Publication
from Agroforestree Database: a tree reference and selection guide
version 4.0
by C. Orwa, A. Mutua, R. Kindt, R. Jamnadass and S. Anthony
Cocos
nucifera L.
Local Names:
Bengali (narikel);
Burmese (on, mak-un); Creole (kokoye); Dutch (cocospalm, coco, cocos,
klapperboom); English (coconut palm, coconut); French (coco, noix de
coco, cocotier, cocoyer, coq au lait); German (Kokospalme,
Kokosnusspalme); Indonesian (kelapa); Italian (cocco); Lao
(Sino-Tibetan) (phaawz); Malay (kelapa); Mandinka (tubab sibo, coc);
Portuguese (coco da India, coco da Bahia, coqueiro de Bahia); Spanish
(cocotero, coco de agua, coco, palma de coco, palmera de coco); Swahili
(mnazi); Tamil (tennai-maram); Thai (ma phrao); Trade name (coconut);
Vietnamese (dùa)
Family: Arecaceae
Botanic
Description
Cocos
nucifera trees
have a smooth, columnar, light grey-brown trunk, with a mean diameter
of 30-40 cm at breast height, and topped with a terminal crown of
leaves. Tall selections may attain a height of 24-30 m; dwarf
selections also exist. Trunk slender and slightly swollen at the base,
usually erect but may be leaning or curved.
Leaves pinnate, feather shaped, 4-7m long and 1-1.5 m wide at the
broadest part. Leaf stalks 1-2 cm in length and thornless.
Inflorescence
consists of female and male axillary flowers. Flowers small, light
yellow, in clusters that emerge from canoe-shaped sheaths among the
leaves. Male flowers small and more numerous. Female flowers fewer and
occasionally completely absent; larger, spherical structures, about 25
mm in diameter.
Fruit roughly ovoid, up to 5 cm long and 3 cm
wide, composed of a thick, fibrous husk surrounding a somewhat
spherical nut with a hard, brittle, hairy shell. The nut is 2-2.5 cm in
diameter and 3-4 cm long. Three sunken holes of softer tissue, called
‘eyes’, are at one end of the nut. Inside the shell is a thin, white,
fleshy layer known as the ‘meat’. The interior of the nut is hollow but
partially filled with a watery liquid called ‘coconut milk’.
The
meat is soft and jellylike when immature but becomes firm with
maturity. Coconut milk is abundant in unripe fruit but is gradually
absorbed as ripening proceeds. The fruits are green at first, turning
brownish as they mature; yellow varieties go from yellow to brown. The
generic name seems to be derived from the Portuguese ‘coco’, meaning
‘monkey’.
Biology
The tall varieties
reproduce by cross-pollination. Male flowers open first, producing
pollen for about 2 weeks. Female flowers are not usually receptive
until about 3 weeks after the opening of the inflorescence, making
cross-pollination the usual pattern. Wind is the main pollinating
agent. Reproduction in dwarf varieties is generally through self -
pollination. Female flowers are receptive about a week after the male
flowers open, both ending at about the same time. C. nucifera flowers
approximately after the 6th year.
Ecology
C. nucifera
is unknown in the wild state. In the coastal areas of the tropics and
subtropics where it is grown, it requires a hot, moist climate and deep
alluvial or loamy soil, thriving especially near the seaboard, but also
considerable distance inland, provided climatic conditions and soil are
suitable. Rocky, laterite or stagnant soils are unsuitable
Biophysical
Limits
Altitude: 520-900 m, Mean annual temperature: 20-28 deg. C, Mean annual
rainfall: 1000-1500 mm
Soil types: C. nucifera
is tolerant to soil variations but its natural preference is for sandy,
well-aerated and well-drained soils. It has considerable ability to
adapt to soils of heavier texture
Documented
Species Distribution
Native:
Brunei, Cambodia, Indonesia, Laos, Malaysia, Myanmar, Philippines,
Singapore, Thailand, Vietnam
Exotic: Argentina, Benin,
Bolivia, Brazil, Burkina Faso, Cameroon, Chad, Chile, China, Colombia,
Cook Islands, Cote d'Ivoire, Ecuador, Fiji, French Guiana, Gambia,
Ghana, Guinea, Guyana, Haiti, India, Jamaica, Kenya, Kiribati, Liberia,
Madagascar, Mali, Marshall Islands, Mauritania, New Caledonia, Niger,
Nigeria, Papua New Guinea, Paraguay, Peru, Samoa, Senegal, Sierra
Leone, Solomon Islands, Sri Lanka, Surinam, Togo, Tonga, Uganda,
Uruguay, US, Vanuatu, Venezuela, Zanzibar
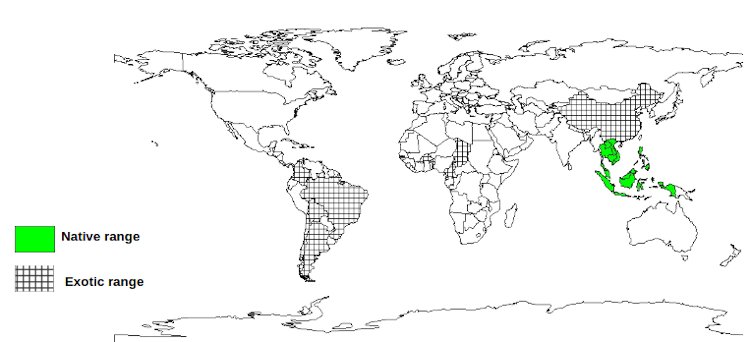
The
map above shows countries where the species has been planted. It does
neither suggest that the species can be planted in every ecological
zone within that country, nor that the species can not be planted in
other countries than those depicted. Since some tree species are
invasive, you need to follow biosafety procedures that apply to your
planting site.
Products
Food:
Copra, the dried coconut endosperm, contains an edible cooking oil
(coconut oil). The apical region of C. nucifera
(‘millionaire salad’) is a food delicacy in areas where it is grown.
Other food derivatives of coconut include coconut chips, coconut jam,
coconut honey, coconut candy and other desserts.
Fodder:
Copra meal and coconut cake, the residues of oil extraction from copra
containing approximately 20% protein, 45% carbohydrate, 11% fibre, fat,
minerals and moisture, are used in cattle feed rations.
Apiculture:
C. nucifera
is an important pollen source for honey production. Where sap is tapped
from unopened inflorescences for toddy-making, many bees drain in the
collecting pots. The honey may be greenish-yellow like the motor oil
and crystal clear if monofloral. Granulation is medium (takes up to 3
months).
Fuel:
The high moisture content of C.
nucifera
wood and the difficulty of splitting it has made it relatively
unpopular as firewood. Coconut shell charcoal is a major source of
domestic fuel in the Philippines. It is also exported to Japan and the
USA. Coconut oil can be used as a substitute for diesel oils, for
electric generating plants and motor vehicles. However, this use is
non-economic in most situations at the present prices of fuel oil.
Fibre:
Three types of fibres are obtained from the coconut husks: mat fibre or
yarn fibre, used in making mats; bristle fibre, used for brush making;
and mattress fibre, used in stuffing mattresses and in upholstery.
Leaflets are used in braiding mats, baskets and hats.
Timber:
C. nucifera
timber has traditionally been used in tropical countries for the
structural framework of houses. Coconut timber taken from the lower and
middle parts of the trunk can be used for load-bearing structures in
buildings, such as frames, floors and trusses. Coconut trunks can be
used for poles, as they have great strength and flexibility. The wood
can also be used for furniture and parquet flooring.
Lipids:
The oil contains fatty alcohol and glycerine used in soaps, detergents,
shampoos cosmetics, pharmaceuticals and explosives.
Alcohol:
Sap from the tender, unopened inflorescence (coconut palm sap) is used
in the producing areas for toddy, or tuba, a beverage obtained by
natural fermentation. Tuba contains 6-7.5% alcohol. The distillation of
fermented coconut toddy yields a spirit called arrack, produced
commercially in Sri Lanka and the Philippines.
Other products:
Coconut-shell flour, obtained from grinding clean, mature coconut
shells to fine powder, is used as a filler in thermoplastic industry
and an abrasive for cleaning machinery. Coconut-shell charcoal may be
processed further into activated carbon that has many industrial
applications, including general water purification, crystalline sugar
preparation and gold purification. The edible mushrooms of the genus Auricularis grow
well on coconut stems and are readily sold in China and elsewhere.
Services
Soil improver:
Burnt husks form a useful sort of potash that is used to fertilize the
trees. The husks also make valuable mulch for moisture conservation in
the dry season and help to suppress weeds.
Ornamental: Planted widely as an ornamental tree.
Intercropping:
Coconut palm is one of the most widely grown tree crops in the tropical
countries. Its growth characteristics are ideal for small production
and also for combining with other crops. The crown morphology and the
relatively wide spacing facilitate the planting of a wide spectrum of
field crops in coconut plantations. It has therefore been intercropped
with cereals (cassava, sweet potatoes, yams) or fruits (bananas,
passion fruit, pineapples and ground nuts) in many countries including
Thailand, India, Sri Lanka, the Philippines etc.
Tree
Management
The
correct planting density depends on soil moisture, variety and soil
type. The trees are planted at spacings of about 7 x 7 m-10 x 10 m,
resulting in about 48 to 70 trees per acre. In home gardens, they
should be planted where they will receive full sun and not be crowded.
The new tree should be watered immediately after planting and
frequently thereafter until it is well established. At least 2.5 cm of
water should be supplied weekly by rain or by irrigation. When cattle
grazing is integrated with coconut cultivation, severe competition for
moisture between palms, bush and grass can be minimized. Mulch applied
to the soil surface around the tree will help retain soil moisture and
restrict weed growth. About 12% of the old trees (over 60 years old)
should be felled each year, resulting in the entire removal of an
initial 94% stand over 8 years. All fronds, logs and stumps should be
removed to control the spread of the rhinoceros beetle (Orycetes rhinoceras, O. moceros).
There is need for a legume cover crop to fix nitrogen to the soil.
Germplasm
Management
Seed
storage behaviour is recalcitrant; 70% of excised embryos survived
desiccation to 14-15% and 44% to 8-9% mc. Cryopreservation techniques
for coconut embryos comprise 4 hours of pretreatment in a medium
containing 600 g/l glucose and 15% glycerol, followed by rapid freezing
and thawing. With this technique, 43% of embryos excised from immature
fruits (7-8 months after pollination) survived 1 month’s cryostorage. A
few cryopreserved embryos produced whole plants, but 33-93% of embryos
excised from mature fruits that had been dried for 4 hours under a flow
hood and then placed in the glucose and glycerol medium detailed above
for 11-20 hours before rapid freezing in liquid nitrogen produced whole
plants with growth delayed by 1-2 months compared with non-frozen
embryos.
Flower pollen oven-dried at 40 deg. C for 40 hours can
be stored over 35% sulphuric acid at room temperatures for 3 weeks.
Pollen can be freeze-dried and stored under vacuum for 1 year or more.
Freeze-dried pollen can be transported at ordinary pollen temperatures
and will retain its viability for 4 months. In ordinary pollen samples,
about 25% may be defective. The best germination was given at 30-35
deg. C with sucrose concentration of 10% and gelatin concentration of
30%.
Pests and
Diseases
Bird
pests include the Hispaniolan woodpecker, which attacks the trunk for
nesting sites and damages immature nuts, and the village weaver, which
strips the leaves for nest building. The nematode Rhadinaphelenchus cocophilus
invades the stem and crown base, causing red-ring disease. More than
100 species of insects afflict the tree, including rhinoceros beetle (Orycetes rhinoceras, O. moceros),
coconut mite (Aceria
guerreronis) and coconut weevil (Rhynchoporus cruentatus).
Other important coleopteran species include Strategus spp.
(attacking the softwood and the heart of the tree), Brontispa spp.
(severely damaging leaves) and leaf miners (Promecotheca spp.)
that render leaves non-functional.
Lethal yellowing is the most important disease of C. nucifera.
Since it was discovered in Key West, Florida, USA, over 200 years ago,
it has crept northward, killing hundreds of thousands of palm trees and
endangering virtually all of the tall coconut palms. Lethal yellowing
is suspected to be caused by a tiny mycoplasma-like organism, visible
only with the aid of an electron microscope. Early symptoms are
premature dropping of coconuts and blackening of flower stalks. The
leaves then turn yellow; beginning with the lower ones and progressing
to the crown, which dies and eventually topples from the tree. The tree
usually dies within 6 months after exhibiting the 1st symptoms. An
injection of the antibiotic oxytetracycline may result in remission of
symptoms within 4 weeks, but additional applications at 4- month
intervals are required to keep the tree alive. Rouging and destruction
of the infected palms and replacement with the resistant Malayan Dwarf
coconut palm is recommended.
Bud rot, caused by the fungus Phytophthora
palmivora, is found in all areas where C. nucifera
is grown. Early symptoms, found on young developing leaves, are brown
sunken spots, yellowing or withering. The leaves turn a light
greyish-brown, becoming darker brown as they collapse at the base. The
infection spreads inward to the bud and outward to surrounding leaves,
which turn yellow and fall off. Young nuts fail to develop and fall,
but those well-formed before infection continue to mature. A
disagreeable odour emanates from the decaying bud. Disease development
most commonly occurs after periods of heavy rains. Early detection is
essential for successful control. Application of a Bordeaux paste to
the buds in early stages may result in recovery if the apical meristem
is not infected. Remove fronds showing early symptoms. Palms showing
advanced symptoms should be removed and destroyed, since they may serve
as a source of inoculum.
Further
Reading
Anderson
GD. 1966. Increasing coconut yield and income on the sandy soils of the
Tanganyika coast. East African Agriculture and Forestry Journal.
32(3):310-314.
Beentje HJ. 1994. Kenya trees, shrubs and lianas. National Museums of
Kenya.
Cobley L.S & Steele W.M. 1976. An Introduction to the Botany of
Tropical Crops. Longman Group Limited.
des Liyange M, Tejwani KG, Nair PKR. 1985. Intercropping under coconut
in Sri Lanka. ICRAF.
Grimwood EB. 1975. Coconut palm products. FAO, Rome.
Hong TD, Linington S, Ellis RH. 1996. Seed storage behaviour: a
compendium. Handbooks for Genebanks: No. 4.
IPGRI.
Katende AB et al. 1995. Useful trees and shrubs for Uganda.
Identification, Propagation and Management for
Agricultural
and Pastoral Communities. Regional Soil Conservation Unit (RSCU),
Swedish International Development Authority (SIDA).
Lanzara P. and Pizzetti M. 1978. Simon & Schuster's Guide to
Trees. New York: Simon and Schuster
Little
EL, Wadsworth FH. 1964. Common trees of Puerto Rico and the Virgin
Islands. Agricultural Handbook. No. 249. US Department of Agriculture.
Washington DC.
MacMillan HF et al. 1991. Tropical planting and gardening. Malayan
Nature Society.
Nicholson B.E, Harrison S.G, Masefield G.B & Wallis M. 1969.
The Oxford Book of Food Plants. Oxford University Press.
Noad T, Birnie A. 1989. Trees of Kenya. General Printers, Nairobi.
Ohler JG. 1984. Coconut, tree of life. Plant Production and Protection
Paper No.57. FAO, Rome.
Perry LM. 1980. Medicinal plants of East and South East Asia :
attributed properties and uses. MIT Press. South East Asia.
Raynor B. 1991. Agroforestry systems in Pohnpei. Practices and
strategies for development. Forestry Development Programme.
Timyan J. 1996. Bwa Yo: important trees of Haiti. South-East Consortium
for International Development. Washington D.C.
Williams R.O & OBE. 1949. The useful and ornamental plants in
Zanzibar and Pemba. Zanzibar Protectorate
|
|
